Demos¶
2D/3D Demos¶
For convenience, a series of demos are included with each distribution of CellOrganizer. These demos show
how to synthesize images from existing models,
how to train new models from raw data, as well as
other functionality, e.g. exporting examples in multiple formats.
To display information about the available demos contained in the distribution, type in Matlab terminal:
>> demoinfo
For example, in Ubuntu
Demos Summary Table¶
This table will let you know if the demo is meant to train a model or synthesize an image. Certains demos have been deprecated and will be removed in future versions of CellOrganizer.
Demo |
Training |
Synthesis |
Other |
Deprecated |
|---|---|---|---|---|
True |
||||
True |
||||
True |
||||
True |
v.2.8.0 |
|||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
v.2.8.0 |
|||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
||||
True |
Report |
|||
True |
Plot |
|||
True |
||||
True |
Info |
|||
True |
||||
True |
Info |
|||
True |
||||
True |
||||
Model |
||||
True |
||||
True |
||||
True |
||||
True |
Plot |
|||
True |
||||
True |
||||
True |
Plot |
|||
True |
Model |
|||
True |
Plot |
|||
True |
||||
True |
Brief Descriptions¶
demo2D00¶
Demo header:
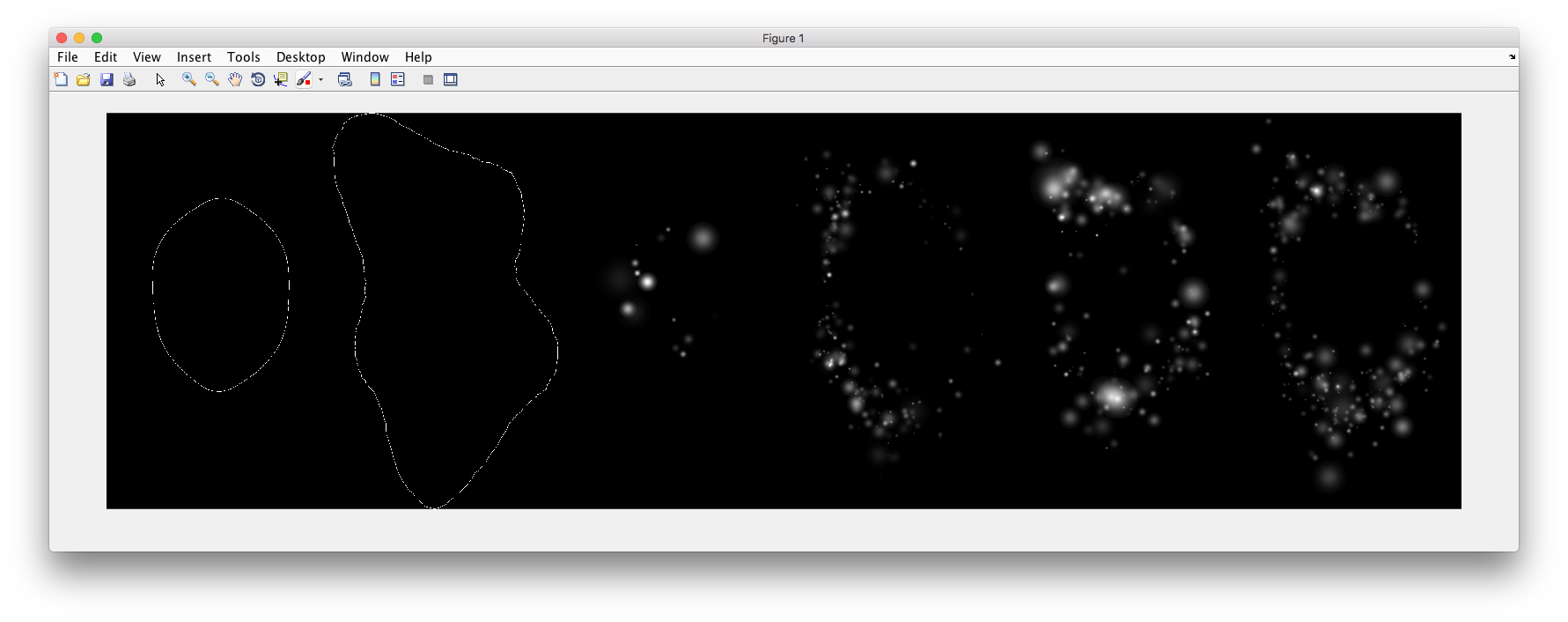
% Synthesize one 2D image with nuclear, cell shape, and vesicular channels
% from all vesicular object models (nucleoli, lysosomes, endosomes, and
% mitochondria) without convolution. The model was trained from the Murphy
% Lab 2D HeLa dataset.
%
% What you need
% -------------
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * one TIFF file with six slices (nuclear, cell shape, nucleolar,
% lysosomal, endosomal, and mitochondrial channels)
Demo output:
demo2D01¶
Demo header:
% Train 2D generative model of the nucleus, cell shape, and lysosome using
% all LAMP2 images in the Murphy Lab 2D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo2D02¶
Demo header:
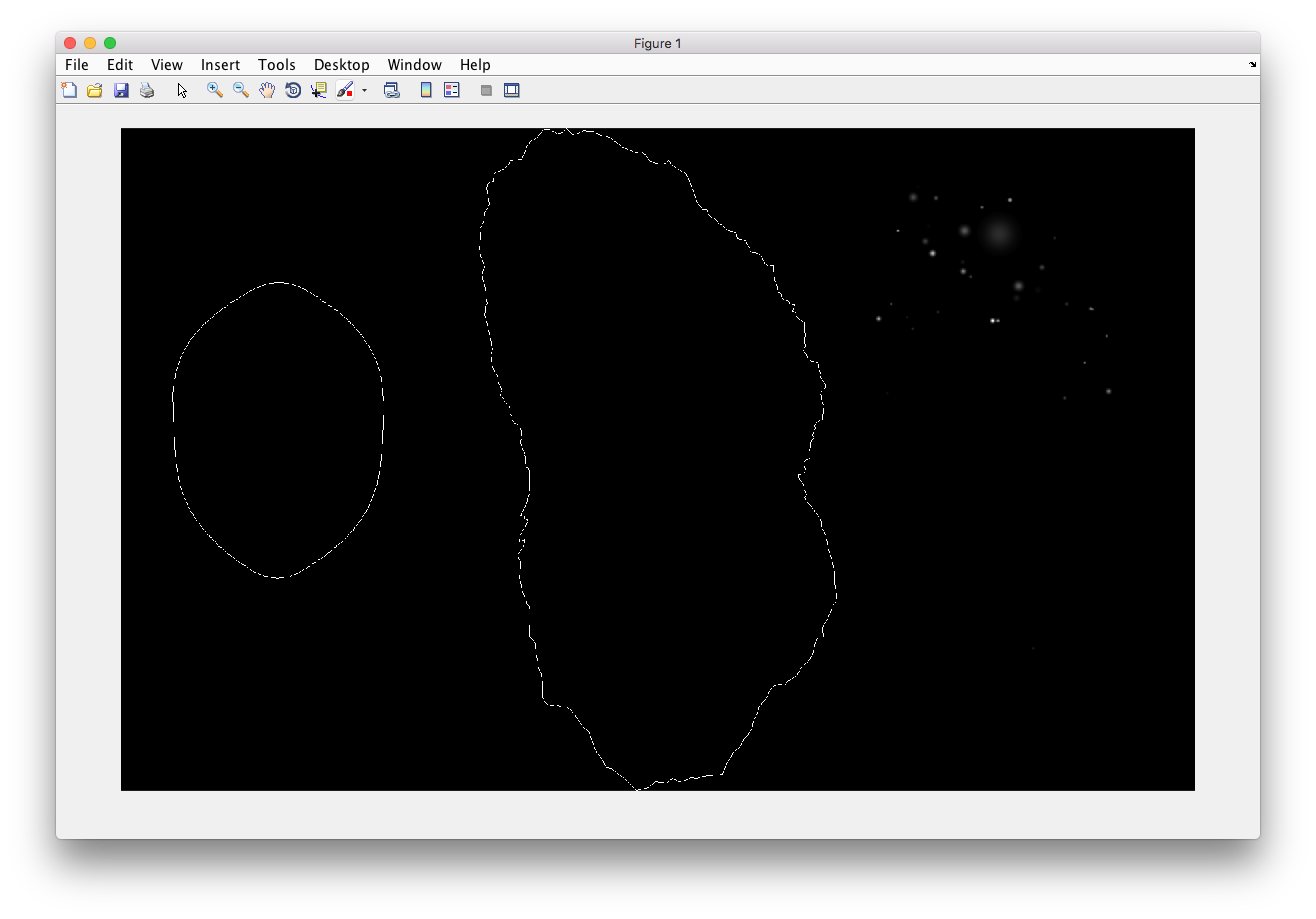
% Synthesize one 2D image with nuclear, cell shape, and lysosomal channels
% from LAMP2 model trained in demo2D01 without convolution.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * one TIFF file with three slices (nuclear, cell shape, and lysosomal
% channels)
Demo output:
demo2D03¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% Train 2D generative model of the nucleus, cell shape, and lysosome using
% all LAMP2 images in the Murphy Lab 2D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo2D04¶
Demo header:
% Train 2D generative diffeomorphic nuclear and cell shape model and a
% lysosomal model using 10 LAMP2 images in the Murphy Lab 2D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo2D05¶
Demo header:
% Train 2D generative pca nuclear and cell shape model using the Murphy Lab 2D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo2D06¶
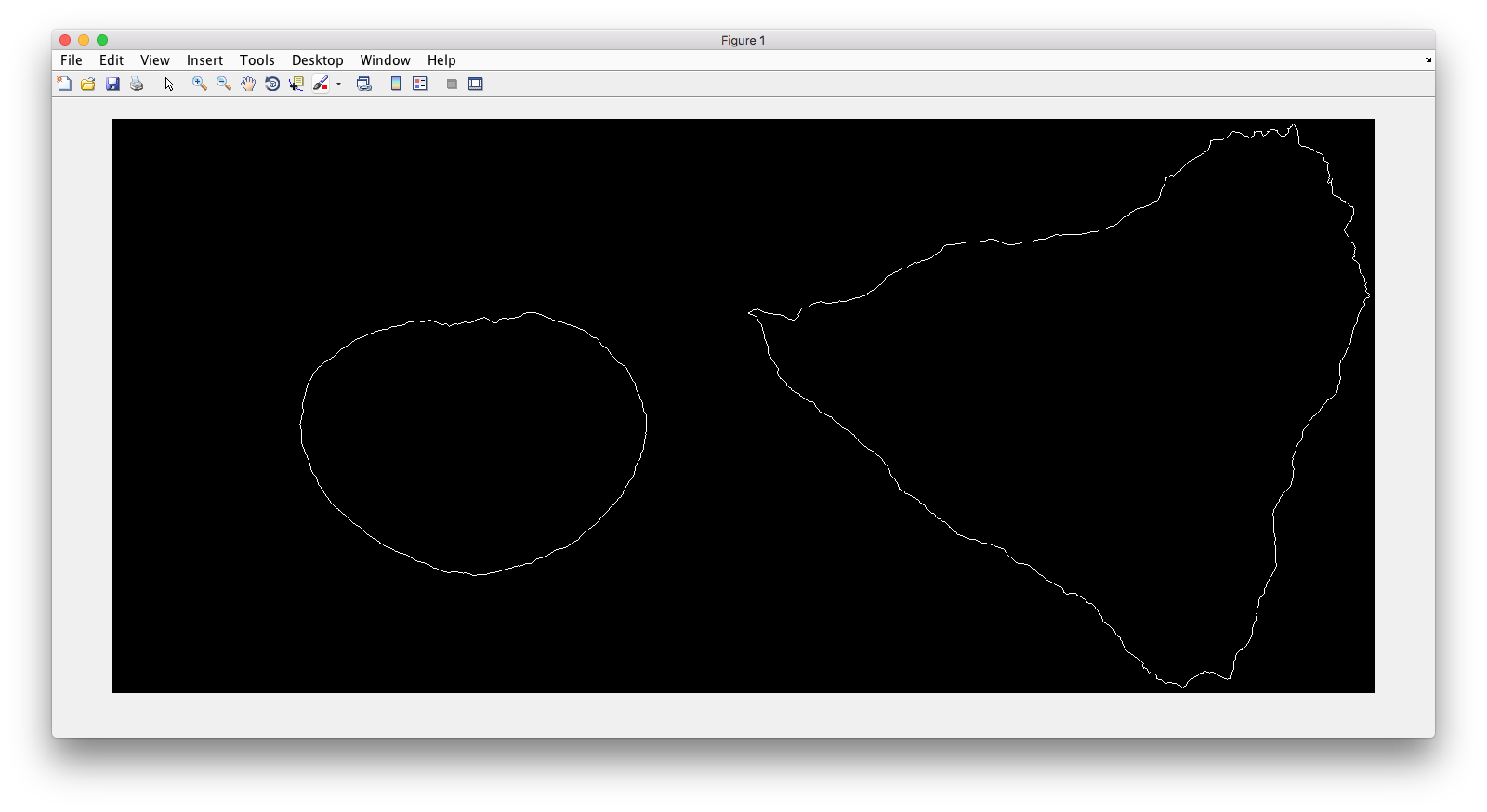
Demo header:
% Reconstruct one 2D image with nuclear, cell shape for PCA model
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * one TIFF file with three slices (nuclear, cell shape, and lysosomal
% channels)
Demo output:
demo2D07¶
Demo header:
% Synthesize one 2D image with nuclear, cell shape with PCA model
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * one TIFF file with three slices (nuclear, cell shape, and lysosomal
% channels)
Demo output:
demo2D08¶
Demo header:
% Train 2D generative pca nuclear and cell shape model using the Murphy Lab
% 2D HeLa dataset and makes a shape space plot
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
% * a shape space plot
demo2D09¶
Demo header:
% Train 2D generative pca nuclear and cell shape model using the Murphy Lab
% 2D HeLa dataset and makes a shape space plot
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
% * a report
demo3D00¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and nucleolar channels
% from nucleolar model with sampling method set to render nucleoli as
% ellipsoids without convolution. The model was trained from the Murphy Lab
% 3D HeLa dataset.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * three TIFF files (nuclear, cell shape, and nucleolar channels)
demo3D01¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and vesicular channels
% from all vesicular object models (lysosomes, mitochondria, nucleoli, and
% endosomes) with sampling method set to render vesicular objects as
% ellipsoids without convolution. The model was trained from the Murphy Lab
% 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * six TIFF files (nuclear, cell shape, lysosomal, mitochondrial,
% nucleolar, and endosomal channels)
demo3D02¶
Demo header:
% Generate surface plot of image synthesized by demo3D00.
%
% Input
% -----
% * three TIFF files (nuclear, cell shape, and nucleolar channels)
% from demo3D00 directory
%
% Output
% ------
% * a surface plot of the synthetic image
demo3D03¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and vesicular channels
% from all vesicular object models (nucleoli, lysosomes, endosomes, and
% mitochondria) with sampling method set to sample vesicular objects from
% Gaussians at density 75 without convolution. The model was trained from
% the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * six TIFF files (nuclear, cell shape, nucleolar, lysosomal, endosomal,
% and mitochondrial channels)
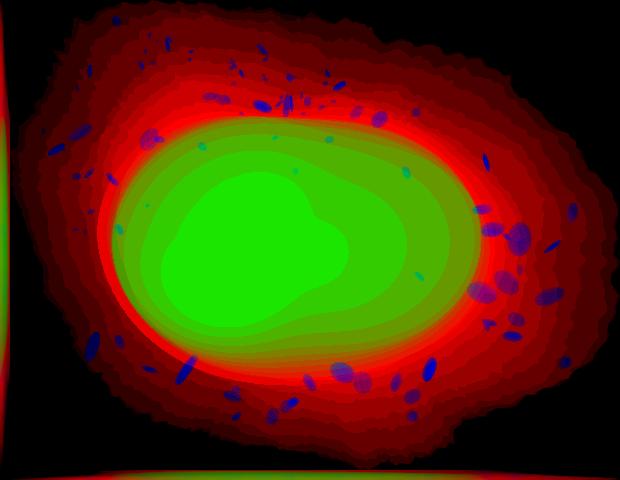
demo3D04¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and vesicular channels
% from all vesicular object models (nucleoli, lysosomes, endosomes, and
% mitochondria) with sampling method set to sample vesicular objects from
% Gaussians at density 75 without convolution. The model was trained from
% the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * six TIFF files (nuclear, cell shape, nucleolar, lysosomal, endosomal,
% and mitochondrial channels)
demo3D05¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and vesicular channels
% from all vesicular object models (nucleoli, lysosomes, endosomes, and
% mitochondria) with sampling method set to sample vesicular objects from
% Gaussians at density 75 without convolution. The model was trained from
% the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * six TIFF files (nuclear, cell shape, nucleolar, lysosomal, endosomal,
% and mitochondrial channels)
demo3D06¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and protein channels
% from all object models (nucleoli, lysosomes, endosomes, mitochondria, and
% microtubules) with sampling method set to render vesicular objects as
% ellipsoids and convolution with point-spread function. The model was
% trained from the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * seven TIFF files (nuclear, cell shape, nucleolar, lysosomal, endosomal,
% mitochondrial, and microtubule channels)
demo3D07¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and protein channels
% from all object models (nucleoli, lysosomes, endosomes, mitochondria, and
% microtubules) with sampling method set to sample vesicular objects from
% Gaussians at a density of 25 and convolution with point-spread function.
% The model was trained from the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * seven TIFF files (nuclear, cell shape, nucleolar, lysosomal, endosomal,
% mitochondrial, and microtubule channels)
demo3D08¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and vesicular channels
% from all vesicular object models (nucleoli, lysosomes, endosomes, and
% mitochondria) with sampling method set to render vesicular objects as
% ellipsoids without convolution. The model was trained from the Murphy Lab
% 3D HeLa dataset.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * single indexed TIFF file which indexes the six TIFF files (nuclear,
% cell shape, nucleolar, lysosomal, endosomal, and mitochondrial channels)
demo3D09¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and lysosomal channels
% from LAMP2 model with sampling method set to render lysosomes as
% ellipsoids without convolution. Also render 2D mean projections along XY,
% XZ, and YZ axes of image. The model was trained from the Murphy Lab 3D
% HeLa dataset.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * three TIFF files (nuclear, cell shape, and lysosomal channels)
% * one projection TIFF file
% * one projection PNG file
demo3D10¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and lysosomal channels
% with object files that can be imported to Blender from LAMP2 model,
% with sampling method set to render lysosomes as ellipsoids without
% convolution. The model was trained from the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * three TIFF files (nuclear, cell shape, and lysosomal channels)
% * three Wavefront OBJ files (nuclear, cell shape, and lysosomal channels)
demo3D11¶
Demo header:
% Train 3D generative model of the cell framework (nucleus and cell shape)
% using the Murphy Lab 3D HeLa TfR dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid model
demo3D12¶
Demo header:
% Train 3D generative model of the nucleus, cell shape, and lysosome using
% 30 LAMP2 images in the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo3D13¶
Demo header:
% Export images synthesized by demo3D01 as object files importable to
% Blender.
%
% Input
% -----
% * a directory of 3D synthetic images
%
% Output
% ------
% * Wavefront OBJ files
demo3D14¶
Demo header:
% Render 2D mean projections along XY, XZ, and YZ axes of images
% synthesized by demo3D00.
%
% Input
% -----
% * a directory of 3D synthetic images
%
% Output
% ------
% * projections of synthetic images as TIFF files
demo3D15¶
Demo header:
% Synthesize one multichannel 3D image from an endosomal model and
% diffeomorphic nuclear and cell shape model. The sampling method was set
% to render endosomes as ellipsoids without convolution. The model was
% trained from the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a valid CellOrganizer model file with a diffeomorphic framework
%
% Output
% ------
% * three TIFF files (nuclear, cell shape, and endosomal channels)
demo3D16¶
Demo header:
% The main idea behind this demo is to show the user they
% can use their own binary images from raw experimental data
% to synthesize protein patterns. This demo uses the CellOrganizer
% method for nuclear and cell segmentation.
%
% The current demo assumes the resolution of the images is the same as
% the resolution of the images that were used to train the protein model.
%
% Input
% -----
% * raw or synthetic images of the nuclear and cell membrane
% * a valid CellOrganizer model file
%
% Output
% ------
% * three TIFF files (cell shape, nuclear, and lysosomal channels)
demo3D17¶
Demo header:
% The main idea behind this demo is to show the user they
% can use their own binary images from raw experimental data
% to synthesize protein patterns.
%
% The current demo assumes the resolution of the images is the same
% as the resolution of the images that were used to train the protein model.
%
% Input
% -----
% * an existing raw or synthetic framework, i.e. one binary multi-TIFF
% file of the nuclear channel and one binary multi-TIFF file of the
% cell membrane
% * the resolution of the latter images
% * a valid CellOrganizer model that contains a protein model
%
% Output
% ------
% * three TIFF files (cell shape, nuclear, and lysosomal channels)
demo3D18¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% Train 3D generative model of the cell framework (nucleus and cell shape),
% using hole-finding to infer both nucleus and cell shape from the supplied
% protein pattern. The 3D 3T3 dataset was collected in collaboration with
% Dr. Jonathan Jarvik and Dr. Peter Berget.
%
% Input
% -----
% * a directory of raw or synthetic protein images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model
demo3D19¶
Demo header:
% This demo uses slml2report to compare the parameters between
% CellOrganizer models and returns a report.
%
% Input
% -----
% * a set of valid CellOrganizer models
%
% Output
% ------
% * a report
demo3D20¶
Demo header:
% Train 3D generative diffeomorphic model of the cell framework (nucleus
% and cell shape) using 10 images Murphy Lab 3D HeLa LAMP2 dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% -------
% * a valid SLML model file
% * a visualization of the shape space
demo3D21¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% Train 3D generative model of the cell framework (nucleus and cell shape),
% using hole-finding to infer both nucleus and cell shape from the supplied
% protein pattern. This is identical to demo3D18 minus scaling the
% images. The 3D 3T3 dataset was collected in collaboration with Dr.
% Jonathan Jarvik and Peter Berget.
%
% Input
% -----
% * a directory of raw or synthetic protein images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model
demo3D22¶
Demo header:
% Synthesizes a protein pattern instance from the synthetic image produced
% in demo3D00.
%
% Input
% -----
% * a synthetic framework
%
% Output
% ------
% * a synthetic image
demo3D23¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% Train 3D generative diffeomorphic nuclear, cell shape, and a
% lysosomal model from all LAMP2 images in the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo3D24¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% This demo converts a sample SBML file to an SBML-spatial instance using
% the "matchSBML" function. This function takes an SBML file, matches the
% compartments in the file with available models and synthesizes the
% appropriate instances.
%
% Input
% -----
% * sample SBML file
%
% Output
% ------
% * valid SBML model
demo3D25¶
Demo header:
% Synthesizes 1 image using a lysosomal model with sampling mode
% set to 'disc', no convolution and output.SBML set to true.
% Results will be three TIFF files, one each for cell boundary,
% nuclear boundary, and lysosomes, in folder "synthesizedImages/cell1"
% Additionally, in the folder "synthesizedImages/" will be a
% SBML-Spatial(v0.82a) formatted .xml file containing constructed solid
% geometry(CSG) primitives for lysosomes and parametric objects for the
% cell and nuclear shapes.
%
% These files can then be read into VCell using the built in importer or
% CellBlender using the helper function provided in this distribution.
%
% Input
% -----
% * valid SBML model
%
% Output
% ------
% * three TIFF files
% * XML file with primitives for lysosomes and parametric objects
demo3D26¶
Demo header:
% This function displays a shape space of some dimensionality. This demo
% uses the model trained in Johnson 2015.
%
% Input
% -----
% * a CellOrganizer diffeomorphic model
%
% Output
% ------
% * a display of the shape space
demo3D27¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% This demo performs a regression between two sets of related shapes (i.e.
% predicts cell shape from nuclear shape) and displays the residuals as in
% Figure 2 of Johnson et al 2015.
%
% Input
% -----
% * models hela_cell_10_15_15.mat and hela_nuc_10_15_15.mat
%
% Output
% ------
% * shape space figure
demo3D28¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape, and nucleolar channels
% from nucleolar model with sampling method set to render nucleoli as
% ellipsoids without convolution. The model was trained from the Murphy Lab
% 3D HeLa dataset.
%
% Input
% -----
% * an existing raw or synthetic nuclear image, i.e. one binary multi-TIFF
% file of the nuclear channel
% * the resolution of the input image
% * a valid CellOrganizer model that contains a cell membrane model
%
% Output
% ------
% * three TIFF files (cell shape, nuclear, and nucleolar channels)
demo3D29¶
Demo header:
% Displays information about a model
%
% Input
% -----
% * valid model
%
% Output
% ------
% * details about the models
demo3D30¶
This demo is deprecated. The demo will be removed in future versions of CellOrganizer.
Demo header:
% This demo illustrates how to sample uniformly at random from a
% diffeomorphic model.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * a random walk
demo3D31¶
Demo header:
% Trains a generative model of microtubules
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid model
demo3D32¶
Demo header:
% Synthesizes 1 image using a lysosomal model with sampling mode
% set to 'disc', no convolution using the object avoidance methods
% Results will be three TIFF files, one each for cell boundary,
% nuclear boundary, and lysosomes, in folder "synthesizedImages/cell1".
%
% Input
% -----
% * valid SBML file
%
% Output
% ------
% * three TIFF files
demo3D33¶
Demo header:
% Synthesize multiple 3D images from a lysosome model, at different resolutions.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% -------
% * multiple instances of the same cell at different resolutions
demo3D34¶
Demo header:
% Synthesize one 3D image with nuclear, cell shape and a vesicular channel.
% This demo exports the synthetic image as an OME.TIFF as well as an
% SBML Spatial instance.
%
% Input
% -----
% * a valid CellOrganizer model
%
% Output
% ------
% * OME.TIFF
% * SBML instance
% * single channel TIF files
demo3D35¶
Demo header:
% This demo uses slml2model to display information from a valid model file
%
% Input
% -----
% * a valid CellOrganizer model
%
% Output
% ------
% * a report
Demo output:
demo3D36¶
Demo header:
% Synthesize multiple 3D images from a lysosome model at different resolutions.
%
% Input
% -----
% * valid lysosomal model
%
% Output
% ------
% * multiple 3D images at different resolutions
demo3D37¶
Demo header:
% This demo exists to illustrate how padding size and window size affect the
% performance of diffeomorphic metric.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% -------
% * a valid SLML model file
demo3D38¶
Demo header:
% Synthesizes 1 image using a lysosomal model with sampling mode
% set to 'disc', no convolution using the object avoidance methods
% Results will be three TIFF files, one each for cell boundary,
% nuclear boundary, and lysosomes, in folder "synthesizedImages/cell1".
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * three TIFF files (nuclear, cell shape, and nucleolar channels)
demo3D39¶
Demo header:
% This demo illustrates how to sample uniformly at random from a
% diffeomorphic model.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * a random walk
demo3D40¶
Demo header:
% Train 3D generative framework model from all LAMP2 images in the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo3D41¶
Demo header:
% Train 3D generative model of the nucleus, cell shape, and lysosome from
% all LAMP2 images in the Murphy Lab 3D HeLa dataset that are either in the
% current directory or in the demo3D11 directory.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * a directory of raw or synthetic lysosome images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo3D42¶
Demo header:
% This demo illustrates using CellOrganizer to train a protein distribution
% model following the approach described in
%
% K. T. Roybal, T. E. Buck, X. Ruan, B. H. Cho, D. J. Clark, R. Ambler,
% H. M. Tunbridge, J. Zhang, P. Verkade, C. Wülfing, and R. F. Murphy (2016)
% Computational spatiotemporal analysis identifies WAVE2 and Cofilin as
% joint regulators of costimulation-mediated T cell actin dynamics.
% Science Signaling 9:rs3. doi: 10.1126/scisignal.aad4149.
%
% The slowest step, which typically takes about 1 min per cell per frame,
% is to align each cell at each time to the standardized template.
% This demo uses 46 cells so it will take about 1 hour on a single core.
%
% Input
% -----
% * image and annotation files for one or more proteins for one or more
% time points
% > the default is to use images from the paper of LAT at time 0 - downloading the
% needed images requires about 4 GB of free disk space
%
% Output
% ------
% * a model for the average concentration in each voxel of a standardized
% cell shape (in demos/LAT_reltime_1.mat)
% * various intermediate results files (in /param and /tmp)
demo3D43¶
Demo header:
% This is the synthesis demo for T cell model.
% The demo takes in two models: one model contains both cell and nuclear
% shape models, and the other contains a T cell protein shape model. Same
% as other synthesis framework, it calls slml2img for the synthesis. The
% meanings of the options are commented in the script.
%
% Input
% -----
% * A protein model with type standardized map halp-elipsoid
% * A framework model the provide the shape of the cell.
%
% Output
% ------
% * one or more set(s) of synthesized images with cell shape and protein
% pattern.
demo3D44¶
Demo header:
% Synthesize a cell shape image from a given constructive_geometry model,
% specifically a half-ellipsoid model.
%
% Input
% -----
% * a list of valid CellOrganizer half-ellipsoid model files
%
% Output
% ------
% * a 3D stacked TIFF file
Demo output:
demo3D45¶
Demo header:
% Train 3D generative model of the cell framework (nucleus and cell shape)
% using the Murphy Lab 3D HeLa TfR dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid model
demo3D46¶
Demo header:
% This is the synthesis demo for T cell model.
% The demo takes in two models: one model contains both cell and nuclear
% shape models, and the other contains a T cell protein shape model. Same
% as other synthesis framework, it calls slml2img for the synthesis. The
% meanings of the options are commented in the script.
%
% Input
% -----
% * A protein model with type standardized map halp-elipsoid
% * A framework model the provide the shape of the cell.
%
% Output
% ------
% * one or more set(s) of synthesized images with cell shape and protein
% pattern.
Demo output:
demo3D47¶
Demo header:
% Combine two generative model files into a single file.
%
% Input
% -----
% * a list of valid CellOrganizer model files
%
% Output
% ------
% * a valid model
demo3D48¶
Demo header:
% This demo illustrates using CellOrganizer to train an updated version of
% protein distribution model following the approach described in
%
% K. T. Roybal, T. E. Buck, X. Ruan, B. H. Cho, D. J. Clark, R. Ambler,
% H. M. Tunbridge, J. Zhang, P. Verkade, C. Wülfing, and R. F. Murphy (2016)
% Computational spatiotemporal analysis identifies WAVE2 and Cofilin as
% joint regulators of costimulation-mediated T cell actin dynamics.
% Science Signaling 9:rs3. doi: 10.1126/scisignal.aad4149.
%
% The updates include:
% 1. one point synapse annotation is allowed as valid input;
% 2. a method is implemented for synapse detection with only providing
% the first time point.
% 3. the method for aligmentment adjustment is implemented.
%
% The slowest step, which typically takes about 1 min per cell per frame,
% is to align each cell at each time to the standardized template.
% This demo uses 46 cells so it will take about 1 hour on a single core.
%
% Input
% -----
% * image and annotation files for one or more proteins for the first
% time point (the default is to use images from the paper of LAT at time 0
% - downloading the needed images requires about 4 GB of free disk space)
%
% Output
% ------
% * a model for the average concentration in each voxel of a standardized
% cell shape (in demos/LAT_reltime_1.mat)
% * various intermediate results files (in /param and /tmp)
demo3D49¶
Demo header:
% This demo illustrates using CellOrganizer to train a protein distribution
% model following the approach described in
%
% K. T. Roybal, T. E. Buck, X. Ruan, B. H. Cho, D. J. Clark, R. Ambler,
% H. M. Tunbridge, J. Zhang, P. Verkade, C. Wuelfing, and R. F. Murphy (2016)
% Computational spatiotemporal analysis identifies WAVE2 and Cofilin as
% joint regulators of costimulation-mediated T cell actin dynamics.
% Science Signaling 9:rs3. doi: 10.1126/scisignal.aad4149.
%
% The slowest step, which typically takes about 1 min per cell per frame,
% is to align each cell at each time to the standardized template.
% This demo uses 46 cells so it will take about 1 hour on a single core.
%
% Input
% -----
% * OMETIFF images with image and annotation files for one or more proteins for one or more
% time points (the default is to use images from the paper of LAT at time 0
% - downloading the needed images requires about 4 GB of free disk space)
%
% Output
% ------
% * a model for the average concentration in each voxel of a standardized
% cell shape (in demos/LAT_reltime_1.mat)
% * various intermediate results files (in /param and /tmp)
demo3D50¶
Demo header:
% Train 3D generative SPHARM-RPDM cell shape model using the Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo3D51¶
Demo header:
% Show shape evolution plot with a trained SPHARM-RPDM model with only cell shape
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
% * a shape space plot
demo3D52¶
Demo header:
% Train 3D generative SPHARM-RPDM nuclear and cell shape model using the
% Murphy Lab 3D HeLa dataset.
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
demo3D53¶
Demo header:
% Reconstruct one 3D image with nuclear, cell shape for SPHARM-RPDM model
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * one TIFF file with three slices (nuclear, cell shape, and lysosomal
% channels)
demo3D55¶
Demo header:
% Show shape space plot with a trained SPHARM-RPDM model
%
% Input
% -----
% * a directory of raw or synthetic nucleus images
% * a directory of raw or synthetic cell shape images
% * the resolution of the images (all images should have the same
% resolution)
%
% Output
% ------
% * a valid SLML model file
% * a shape space plot
demo3D56¶
Demo header:
% This demo illustrates using CellOrganizer to train an updated version of
% protein distribution model following the approach described in
%
% K. T. Roybal, T. E. Buck, X. Ruan, B. H. Cho, D. J. Clark, R. Ambler,
% H. M. Tunbridge, J. Zhang, P. Verkade, C. Wülfing, and R. F. Murphy (2016)
% Computational spatiotemporal analysis identifies WAVE2 and Cofilin as
% joint regulators of costimulation-mediated T cell actin dynamics.
% Science Signaling 9:rs3. doi: 10.1126/scisignal.aad4149.
%
% The updates include:
% 1. one point synapse annotation is allowed as valid input;
% 2. a method is implemented for synapse detection with only providing
% the first time point.
% 3. the method for aligmentment adjustment is implemented.
%
% The slowest step, which typically takes about 1 min per cell per frame,
% is to align each cell at each time to the standardized template.
% This demo uses 46 cells so it will take about 1 hour on a single core.
%
% Input
% -----
% * OMETIFF images with image and annotation files for one or more proteins for the first
% time point (the default is to use images from the paper of LAT at time 0
% - downloading the needed images requires about 4 GB of free disk space)
%
% Output
% ------
% * a model for the average concentration in each voxel of a standardized
% cell shape (in demos/LAT_reltime_1.mat)
% * various intermediate results files (in /param and /tmp)
demo3D57¶
Demo header:
% This demo illustrates using CellOrganizer to show protein enrichment plot
% for certain regions of the 3D T cell following the approach described in
%
% K. T. Roybal, T. E. Buck, X. Ruan, B. H. Cho, D. J. Clark, R. Ambler,
% H. M. Tunbridge, J. Zhang, P. Verkade, C. Wülfing, and R. F. Murphy (2016)
% Computational spatiotemporal analysis identifies WAVE2 and Cofilin as
% joint regulators of costimulation-mediated T cell actin dynamics.
% Science Signaling 9:rs3. doi: 10.1126/scisignal.aad4149.
%
% Input
% -----
% * a set of t cell models with different time points
%
% Output
% ------
% * Plots of enrichment for different purpose
demo3D58¶
Demo header:
% demo3D58
%
% Synthesize one 3D image with nuclear, cell shape and a vesicular channel.
%
% Input
% -----
% * a valid CellOrganizer model file
%
% Output
% ------
% * three TIFF files (nuclear, cell shape, and nucleolar channels)
demo3D59¶
Demo header:
% demo3D59
%
% Synthesize one 3D image with nuclear, cell shape and a vesicular channel.
% This demo exports portions of the synthetic image as SBML Spatial instances.
%
% Input
% -----
% * a valid CellOrganizer model
%
% Output
% ------
% * SBML instance
% * single channel TIF files
demo3D60¶
Demo header:
% demo3D60
%
% Synthesize one 3D image with nuclear, cell shape and a vesicular channel.
% This demo exports portions of the synthetic image as SBML Spatial instances.This
% demo also produces a valid VCML file.
%
% Input
% -----
% * a valid CellOrganizer model
%
% Output
% ------
% * SBML instance
% * VCML file
% * single channel TIF files